COVID-19 (Coronavirus Disease) started with its first wave in December 2019 in China and soon spread to the rest of the world with a large number of cases in the first half of 2020 in several countries across all continents. Subsequently, many countries saw the 2nd COVID wave with mutant variant strains of the virus, like the Delta strain in the first half of 2021. The 3rd wave of COVID starting Dec 2021 onwards was seen with the Omicron variant, spreading across the world.
The information in this article pertains broadly to COVID, its symptoms, and diagnosis as seen from 2020-2022.
For specific information about COVID variants and Omicron, read:
Current and Past COVID variants
SYMPTOMS AND DISEASE
COVID-19 (coronavirus disease) is an illness caused by the virus designated 2019-nCoV (now called SARS-CoV-2), which is a novel strain of the coronavirus group first seen in China in December 2019. This illness is similar to viral flu and therefore is called ILI (influenza-like illness). From the time of getting infected, it can take from 2-10 days (incubation period) to manifest definite and recognizable symptoms. The disease course consists of the first week of viremia (viral replication and shedding) and the second week of inflammation (due to the body’s immune response).
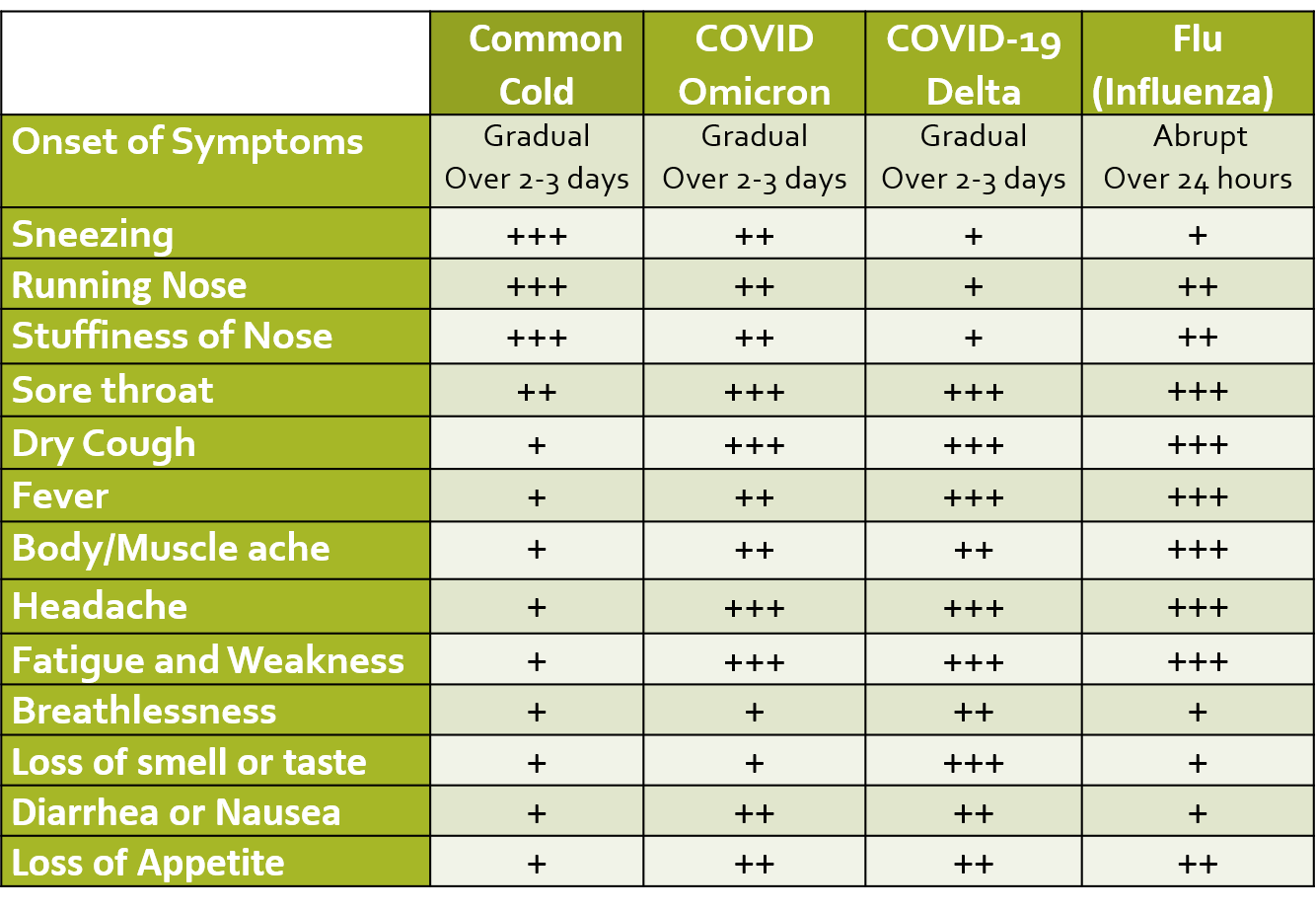
ILI is defined as fever of 38 deg C or more, and cough with onset within the last 10 days. Presenting symptoms of COVID include fever, dry cough, and sore throat which may be accompanied by fatigue, body ache, headache, eye pain or sore eyes, nasal congestion, running nose, nausea/vomiting, loss of appetite or diarrhea. These symptoms are usually very mild in the case of the Omicron variant as compared to other variants like Delta. Many infected patients are often asymptomatic. Loss of smell and taste (salt and sweet), are characteristic signs especially in otherwise asymptomatic people with suspected exposure to COVID, however, these symptoms are not seen in cases of the Omicron variant.
In about 4-5 days, the virus can spread and cause damage to the lower respiratory tract (bronchi and lungs) and decrease blood oxygen saturation. This manifests as breathlessness in addition to the other symptoms. Rarely neurological symptoms like dizziness, decreased alertness, disorientation, fits, or a stroke have also been seen.


COMPLICATIONS AND MORTALITY
Causes of death in COVID include ARDS (acute respiratory distress syndrome) which is respiratory failure resulting from massive lung inflammation and scarring (fibrosis), decreased oxygen in the blood, and/or sepsis leading to multi-organ failure (including heart and kidney failure). More recent research has pointed towards involvement and damage to the endothelium (the inner lining of blood vessels) as the possible cause for severe disease and mortality. Endothelial damage causes an increased risk of blood clotting (thrombosis), as well as adverse cardiovascular effects.
The overall mortality rate from COVID-19 in the first wave was around 2-3% (<1% below 50 years of age). The risk of serious disease, complications, and death is higher and seen far more in older patients (>60 years) and those suffering from other underlying medical illnesses like diabetes, hypertension, cancers, and diseases of the airway/lungs, heart, kidney, liver or immune system. The risk of complications, hospitalization, and death is much lower with the Omicron COVID variant.
EPIDEMIOLOGY
The WHO in February 2020, had designated COVID-19 as a “public health emergency of international concern” (PHEIC) and labeled it a pandemic indicating that international and collaborated action will be required to tackle this infection. During the course of the pandemic in 2020-22, more than 500 million people have been infected globally to date. More than 90% of people have mild symptoms or are recovered at a given time. Post recovery weakness, reduced work capacity, and shortness of breath have been seen for prolonged periods in some patients due to damage caused to the lung and blood vessels.
There were more than 2 million deaths globally in the first COVID wave seen in 2020. However, the 2nd wave of resurge of COVID cases was seen in several parts of the globe in 2021 taking the mortality toll to 4-5 million. The 3rd wave in 2022 due to Omicron showed high numbers in terms of infected people but far lower hospitalization and mortality rates compared to the previous 2 waves.
It is still uncertain as to how long immunity lasts after being infected with the coronavirus. There have been cases of re-infection within 3-6 months in cases who were COVID positive with mild or no symptoms. Infection rates, transmission, and symptomatic COVID in children have been seen to be very low.
By the end of 2020, vaccines have been developed for COVID, and immunization is now available in most countries. Many countries have completed the primary 2-dose vaccination in adults, even started vaccinating children, and giving booster doses.
By May 2023, WHO did not consider COVID as a public health emergency of international concern, anymore.
TRANSMISSION AND SPREAD
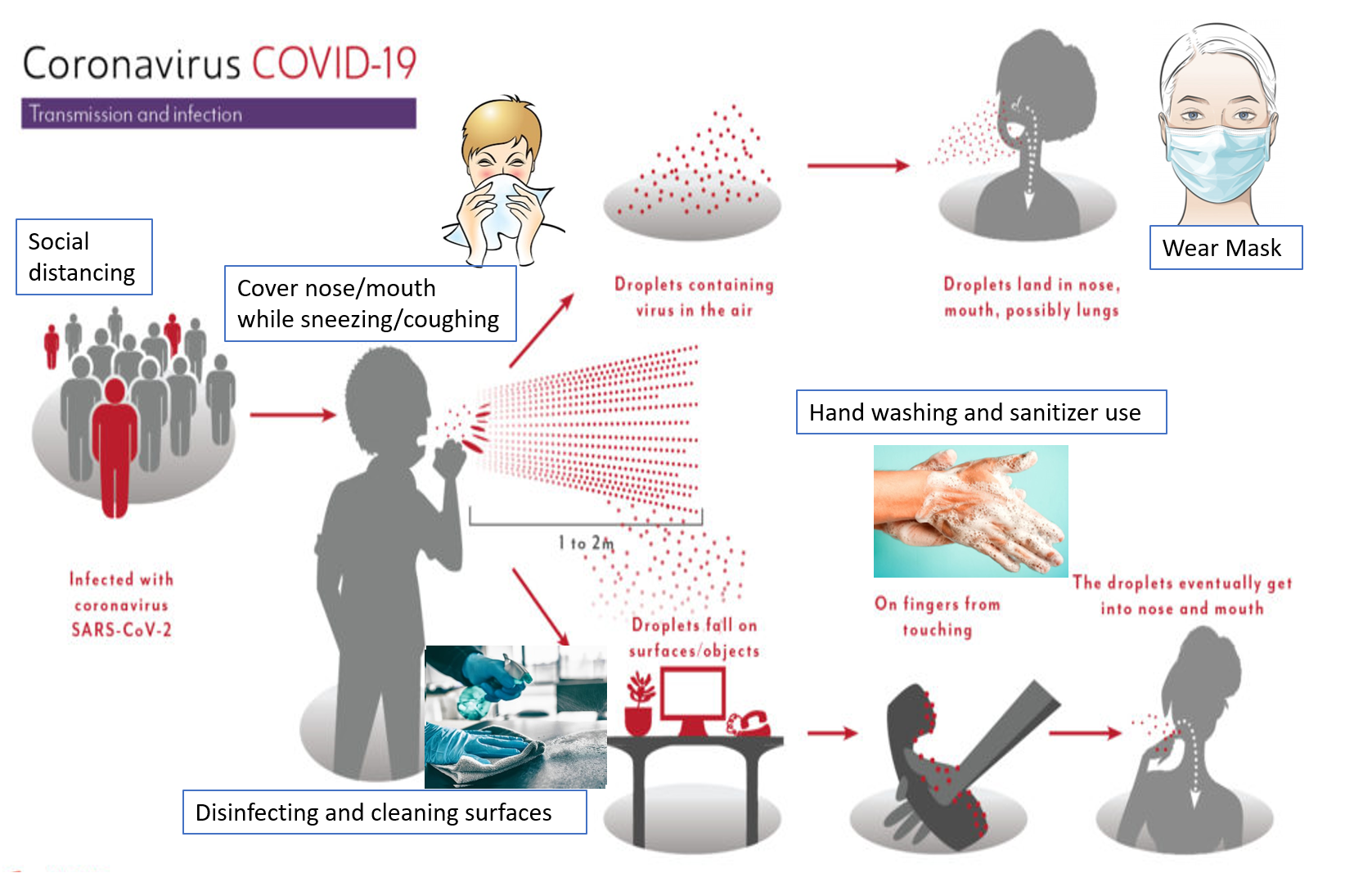
The SARS-CoV-2 is transmitted between humans through droplets and aerosols (smaller droplets usually<5 microns) emitted from infected individuals through coughing, sneezing, spitting, or talking. One gets exposed to these droplets by coming in direct contact with infected persons, infected surfaces, and suspended droplets in the air especially when at close proximity (about 1-2 meter/ 3-6 feet) of an infected patient. How far the droplets travel depends on many factors. Aerosols being smaller and lighter travel further and remain suspended longer in the air than larger droplets, however, aerosols also have less viral load per particle and can diffuse easily. A higher force of expulsion (as in sneezing and coughing, as compared to talking or breathing out), and more wind velocity can increase the degree of droplet transmission. The virus can remain on metal surfaces, plastic, and fabric for up to 12 hours. The Omicron variant has shown the highest transmission and fastest spread.
Every country has taken immense measures to curtail the spread of COVID-19 and limit the number of cases and deaths. Measures include restricting flights or and pre-testing flyers, limiting public transport, monitoring or closing public places and institutions, imposing periodic lockdowns and curfews, disinfecting and sanitizing common areas, and stepping up testing units and hospital facilities. However, the most important thing is for the people to follow and adhere to the advisories and recommendations of their respective governments, and take the required precautions and care without panicking.
Read – COVID-Prevention, Precautions and Care


TESTING
DETECTION OF VIRUS OR ANTIGEN
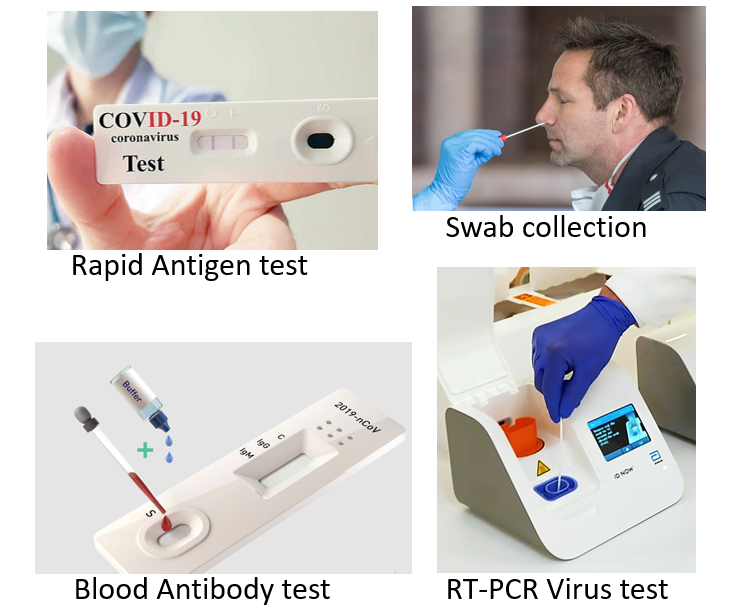
These include tests that detect the genetic material of the virus NAAT (nucleic acid amplification test) and those which detect antigens or surface proteins of the virus (antigen tests). A cotton swab sample from the nose-throat and/or mouth-throat junction (nasopharyngeal and/or oropharyngeal swab) is taken to test for the presence of the virus (antigen) in an individual. A person is said to be positive if the presence of the COVID-19 virus is confirmed in the nose or throat swab sample. The following are some of the tests used:
RT-PCR (Real-time Reverse Transcription Polymerase Chain Reaction) tests work by amplifying genetic fragments of the viral RNA with the help of a process called reverse transcription (conversion to DNA). Thereafter primers are added which bind to the selected DNA sequences, along with a fluorescent probe that helps in real-time antigen detection. The Ct value (cycle threshold) can give some indication about viral load and how infective a person is. Ct values above 24 usually imply a low risk of transmission. Every affected country has set up designated testing centers and reference standard laboratories for RT-PCR testing for COVID-19. It usually takes 24-36 hours for getting the test results. Recently RT-PCR by dry swabs has been approved which omit the need for viral transport medium and the step of RNA extraction/isolation. RT-PCR with gargle sample instead of swab has also been recently approved. These can decrease both cost and the turnaround time by almost 50%. RT-PCR has the highest sensitivity among all antigen tests and is the gold standard test for declaring a person COVID negative.
CB-NAAT (Cartridge-based Nucleic acid Amplification Test) detects the E (envelope) gene as well as the key replication enzyme RNA-dependent RNA polymerase of the virus. TrueNAT is the portable form of CB-NAAT used in labs. TrueNAT is compact and uses a cartridge and microchip. Therefore it gives faster results (in 1-2 hours), is cheaper, convenient to transport, and is very useful in interior areas, to set up kiosks, camps and drive-through testing facilities.
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) has recently been approved with high sensitivity and specificity (96% and 98% respectively) and uses a specially adapted Cas9 protein to successfully detect the virus. The paper-strip uses cutting-edge CRISPR gene-editing technology to identify and target this genetic material of SARS-CoV-2 and can give results in less than an hour.
Virus detection tests done by RT-PCR, CRISPR, TrueNAT, or CBNAAT are considered equivalent and do not require further confirmatory tests.
Rapid Antigen Tests (RAT), now available as test kits even for home use (COVISELF), are point-of-care tests giving results immediately in a few minutes. They are most economical in price and are available in the form of convenient test strip packs. The test is performed for quick and mass screening, as home testing, or in institutes, large commercial establishments, and organizations. RATs also help increase detection rates in containment zones, hot spots, and migrant clusters. RAT act by detecting the spike surface protein of the coronavirus (which facilitates its entry into human cells). RATs are less sensitive than the conventional RT-PCR and though a positive result is taken as confirmed, a negative result could be false if viral load is low or the disease stage is early, and so will need confirmation by RTPCR. If the person is symptomatic, but negative on the RAT, a conventional RT-PCR should be performed.
COVIRAP is a recently introduced rapid test in India that uses isothermal amplification techniques (IAT) as alternatives to PCR to rapidly and efficiently accumulate nucleic acid sequences at a constant temperature. The test is read with colored lines on treated paper strips dipped into the processed sample to detect the virus. It is economical, faster (under 1 hour), portable, reusable (many tests from one unit), and can be handled by relatively unskilled operators on the field. It can detect low viral loads and can be useful in early stages of infection, preventing the uncontrolled spread and timely isolation. It has 94% sensitivity and 98% specificity of that of RT-PCR.
The Indian Council of Medical Research (ICMR) has recently approved 2 new test kits. CoviDelta diagnostic kit developed indigenously in India detects all current variants of COVID-19 and flags presumptive Delta and Omicron variants in a single test and targets the downstream Spike L452R mutation which is present in the ‘Delta’ lineage but absent in all the Omicron sub-lineages. OmiSure RT-PCR test kit approved detects the Omicron variant in 90 minutes by using a dual strategy of both S gene dropout or S gene target failure (SGTF) and the S gene mutation amplification, targeting 3 genes, which detects mutations explicitly in the S gene. Another test called Krivida, approved in India can detect Omicron and its sublineages through a 4 gene strategy in 45 minutes and differentiate it from other variants. Both OmiSure and Krivida are approved in India.
Who should be tested?
People with the presence of ILI symptoms:
When community transfer is evident, ideally all people showing any ILI symptoms of fever, sore throat, cough, body pain, fatigue, etc, especially with the loss of smell/taste (absent in Omicron), should be tested for COVID. However, the below categories of people must definitely be tested when showing likely COVID symptoms.
- Has a household COVID positive case
- Had known or possible contact with a lab-confirmed COVID case
- Had contact with a person who returned from international travel to a COVID affected country in the last 14 days
- Is residing in a hotspot, containment zone, or building with COVID cases
- Is a migrant and returned to home-town or village in the last 14 days
- Is hospitalized and develops ILI, or has SARI (severe acute respiratory infection)
- Is a COVID healthcare worker (HCW)/front-line worker
People without symptoms (asymptomatic):
Recommendations for testing asymptomatic people vary depending on the area and rise in cases.
People who possibly had contact with a lab-confirmed COVID positive case and are asymptomatic should be in 14-day self-isolation to observe for symptoms. (This has been shortened to 5-7 days in some countries with respect to the Omicron variant). The same has also been advised for asymptomatic people who have traveled in the last 14 days to a COVID-affected country especially those countries identified with COVID variants (or have had contact with a symptomatic person who had traveled in the last 14 days to such countries). These people are tested when they develop ILI symptoms or can get themselves tested after 3-5 days of isolation if no symptoms develop.
People living in the same household as a COVID positive case are considered high-risk contacts (HRC), and should be in strict isolation for 10-14 days. Direct household contacts as well as those contacts having co-morbid conditions like diabetes, hypertension, cancer, or elderly>65 years, should be tested immediately on the onset of any possible ILI symptom, or at day 5 after their COVID positive contact developed symptoms.
Health care workers handling COVID-positive patients should be tested if they develop symptoms. If a healthcare worker has handled a COVID positive case without adequate protection, he/she may be tested even if asymptomatic.
Asymptomatic people are to be tested for COVID if they are due to undergo any hospital procedure or surgery. However, no emergency care should be denied to any patients on grounds of waiting for or getting positive test results. Universal precautions, personal protective equipment (PPE), isolation, and hygienic measures should be taken throughout the procedure and patient’s hospital stay.
Asymptomatic people may be screened with rapid antigen tests (RAT) at public areas in hot spots or places where case clusters, or a spurt in cases is being seen. RAT is also available for self-screening at home. Testing by RT-PCR is also a requirement for international travel to certain countries. However, in 2022, mandatory testing and isolation for asymptomatic people have been relaxed by most countries, especially if vaccinated.


ANTIBODY TEST
These tests work by testing antibodies (IgM and IgG) to SARS-CoV-2 proteins in the blood (serological test).
Antibody tests used commercially test for binding antibodies against S (spike surface protein) and N (nucleocapsid protein) of the coronavirus. Two types of these antibodies may be present: IgM (denotes recent infection) or IgG (denotes past infection or vaccination). The techniques used are either ELISA or ECLIA, in which purified S/N proteins of SARS-CoV-2 inactivated virus are added to the blood sample along with detecting reagents. These are also available as rapid tests.
The other type of antibody tests used in research and in clinical trials for vaccines (but not generally approved for commercial use) test for neutralizing antibodies. These tests determine the actual functional ability of antibodies to prevent virus infection by growing the sample with the virus in laboratory cultures. These include the plaque-reduction neutralization test (PRNT) and microneutralization test (MNT).
Antibody tests may be useful for screening large population groups in areas with a high number of cases to detect the extent of disease spread, and how much population has been exposed to the virus. A positive (reactive) test, can imply both active symptomatic or asymptomatic infection, as well as past exposure and immunity, developed to the virus naturally or by vaccination.
OTHER TESTS
High-Resolution Chest CT scan (HR-CT):
This may be used for the detection of COVID and to assess disease severity and complication risk. HR-CT can detect COVID even when RT-PCR is negative, and helps to assess the severity of lung involvement accurately.
A CT severity score of COVID-associated pneumonia is given based on the lung involvement seen. Each lobe (3 right and 2 left) could be awarded a CT score from 0 to 5, depending on the percentage of the involved lobe: score 0 (no involvement); score 1 (<5% involvement); score 2 (5-25% involvement); score 3 (26-49% involvement); score 4 (50-75% involvement); score 5 (>75% involvement). A score of 7 or less, 8-17, and 18 or more signify mild, moderate, and severe pneumonia respectively.
In addition, another score called CO-RADS may also be given that signifies the degree to which the pattern of lung involvement and changes are typical and suggestive of COVID. A CO-RAD score of 5 is highly diagnostic of COVID, while lower the score, the possibility of other infections or lung conditions increases. CO-RADS of 6 is given when the RT-PCR for COVID is positive along with typical characteristic changes in the lung.
X-ray with AI assessment is also available for COVID diagnosis in some countries.
Blood inflammatory markers: The risk of severe disease, complications, and mortality is further assessed by an increase in levels of certain blood markers like neutrophil/lymphocyte ratio, and CRP (C reactive protein). Other tests like D-dimer (signifies risk of thrombosis), ferritin, lactate dehydrogenase, cardiac troponin 1 and cytokine IL-6 are of relevance and importance in hospitalized patients to assess severity, monitoring progression, need for certain treatments, and prognosis.
Read: COVID TREATMENT AND MEDICAL CARE (Levels of Care, Medicines for treating COVID, and Vaccines)
Read: Types of Coronavirus and SARS-CoV-2 (COVID) variants
Also read-
5 Important points of Preventive Care for Coronavirus and COVID-19
Differentiating Breathlessness due to COVID/Flu from that due to Anxiety
Common Cold and Flu- Know the difference, effective care and 10 alert signs
For any query or additional information, please leave a message below this article and be assured of a response soon.
References:
Ministry of Health and Family Welfare, India