Updated Nov 2024
Antibiotics are a class of medicines used to treat Infections.
We are all familiar with the term infection which essentially makes us sick in some way due to germs invading our body. These germs like bacteria, fungi, viruses, and parasites are called micro-organisms as they are seen under a microscope. Barring a few fungi and parasites, these organisms are not visible to the naked eye. Today the term antibiotics has become synonymous with antibacterial medicines used to kill or inhibit the growth of bacteria, while antimicrobials include medicines against all micro-organisms: antibiotics, antifungals, antivirals and antiparasitic drugs.
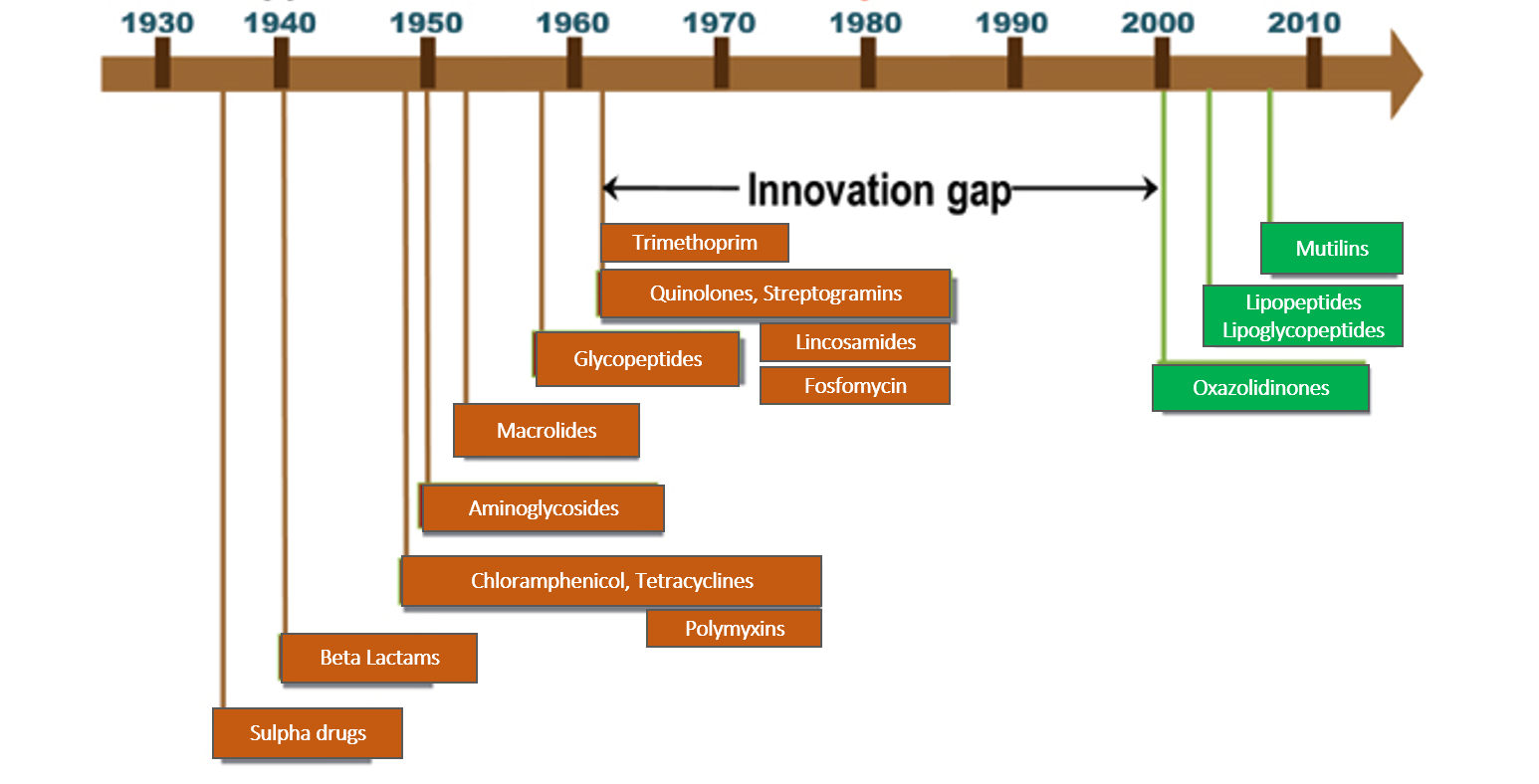
In this section, the antibiotics (antibacterial drugs) have been reviewed. The last new antibiotic launch was in July-Aug 2019 with lefamulin, a drug of the class called mutilins (also called pleuromutilins). Subsequently, new antibiotic classes and drugs are under various phases of development and research. Nomenclature and class are based on chemical structure and the site and mechanism of action on the bacteria.


HOW DO ANTIBIOTICS WORK
Human beings in their constant fight against infections developed weapons called ‘antibiotics’ way back in the 1930s with the discovery of penicillin and sulfa drugs. Actually, antibiotics were substances derived from micro-organisms that could destroy or inhibit the growth of other micro-organisms in a high dilution by a mechanism that would not harm human cells. Today, we have developed several synthetic antibiotics as well in labs. The key to understanding how antibiotics work is to exploit the differences between the cell structure of bacteria and humans.
Antibiotics usually act on the following parts of the bacterial cell-
CELL WALL is an outer covering of the cells of bacteria, plants, and fungi, which is absent in human cells. The cell wall in bacteria consists of a cross-linked substance called ‘peptidoglycan’ (PG) which keeps the cell protected and strong. So preventing PG cross-linking or PG synthesis is a key way in which some classes of antibiotics act to kill bacteria (beta-lactams, glycopeptides, and fosfomycin).
CELL MEMBRANE is a structure that is universally present in all living cells but differs in its composition between organisms. The bacterial cell membrane does not have the ‘sterols’ which are like strong pillars preventing the membrane from disrupting easily. Therefore, some antibiotics can insert themselves into bacterial cell membranes and disrupt it thereby killing the bacteria. (polymyxin). Other drugs act by disrupting the cell membrane by creating an ion-conducting channel and inhibiting protein synthesis (lipopeptides).
Lipoglycopeptides have a dual action of inhibiting bacterial cell-wall synthesis by binding to peptidoglycan precursors as well as ionic disruption of the cell membrane.
RIBOSOMES are small round structures within all living cells that synthesize vital cell proteins. The ribosome type in bacterial cells (50S/30S) is different from those of humans (60s/40S). So antibiotics inhibiting protein synthesis by acting on the 30S or 50S part of ribosomes destroy the bacteria thereby sparing human cells. (30S inhibition – tetracyclines and aminoglycosides; 50S inhibition-macrolides, chloramphenicol, lincosamides, streptogramins, oxazolidinones, and mutilins)
DNA in human cells is organized into chromosomes and well contained inside an enveloped structure called the nucleus. The bacterial DNA is loose and unprotected in an open space called the nucleoid. So bacterial DNA is the target of some antibiotics which prevent or block enzymes of DNA replication and function, thereby inhibiting the growth and multiplication of the bacteria (quinolones, anti TB drug rifampicin). Some antibiotics can directly damage bacterial DNA (metronidazole, nitrofurantoin).
Bacteria also synthesize their own folic acid which is vital for its functioning, while humans get it from the diet. Therefore, inhibiting folic acid synthesis is also one of the mechanisms of action as seen in case of sulpha drugs/trimethoprim.


WHY DOES ONE ANTIBIOTIC NOT ACT ON ALL BACTERIA
You may have often heard the term gram-positive and gram-negative bacteria. The difference lies in the gram-negative ones having an additional fatty layer (called LPS-lipopolysaccharide) outside the PG layer of the cell wall. They stain red with a chemical stain called Gram’s stain. (common bacteria are E coli, Klebsiella, Salmonella, Shigella, Proteus, Hemophilus, Neisseria, Pseudomonas, Moraxella, Vibrio, and Acinetobacter). The bacteria that don’t have this LPS layer and have only the PG layer, stain blue and are called Gram-positive (common bacteria are Staphylococcus, Streptococcus, Pneumococcus, Enterococcus).
So evidently antibiotics have to work harder for the gram-negative bacteria as they have an additional tough layer to cross, so only some of the antibiotics have this ability (broad-spectrum – act on both gram-positive and negative bacteria). Chemical modifications are made to help transport across this LPS layer (extended-spectrum antibiotics).
There are also some bacteria called ‘atypical’ as their cell wall is either absent or has a different composition lacking significant PGs in it (the bacteria called Mycobacterium causing TB, or some of the bacteria causing pneumonia, like Legionella, Mycoplasma and Chlamydia). So antibiotics have to be selected that act within the cell and not on the bacterial cell wall.
The antibiotics mentioned so far act best in the presence of oxygen so are effective for most bacteria that use oxygen to survive (aerobes). However, some bacteria survive mainly without the presence of oxygen (anaerobic bacteria). Antiparasitic (antiprotozoal) drugs metronidazole (or tinidazole, secnidazole, ornidazole) are used for these bacteria as these drugs act by generating oxygen free radicals (reactive oxygen species -ROS) that are destructive to anaerobic bacterial cells.
HOW ARE ANTIBIOTICS TAKEN?
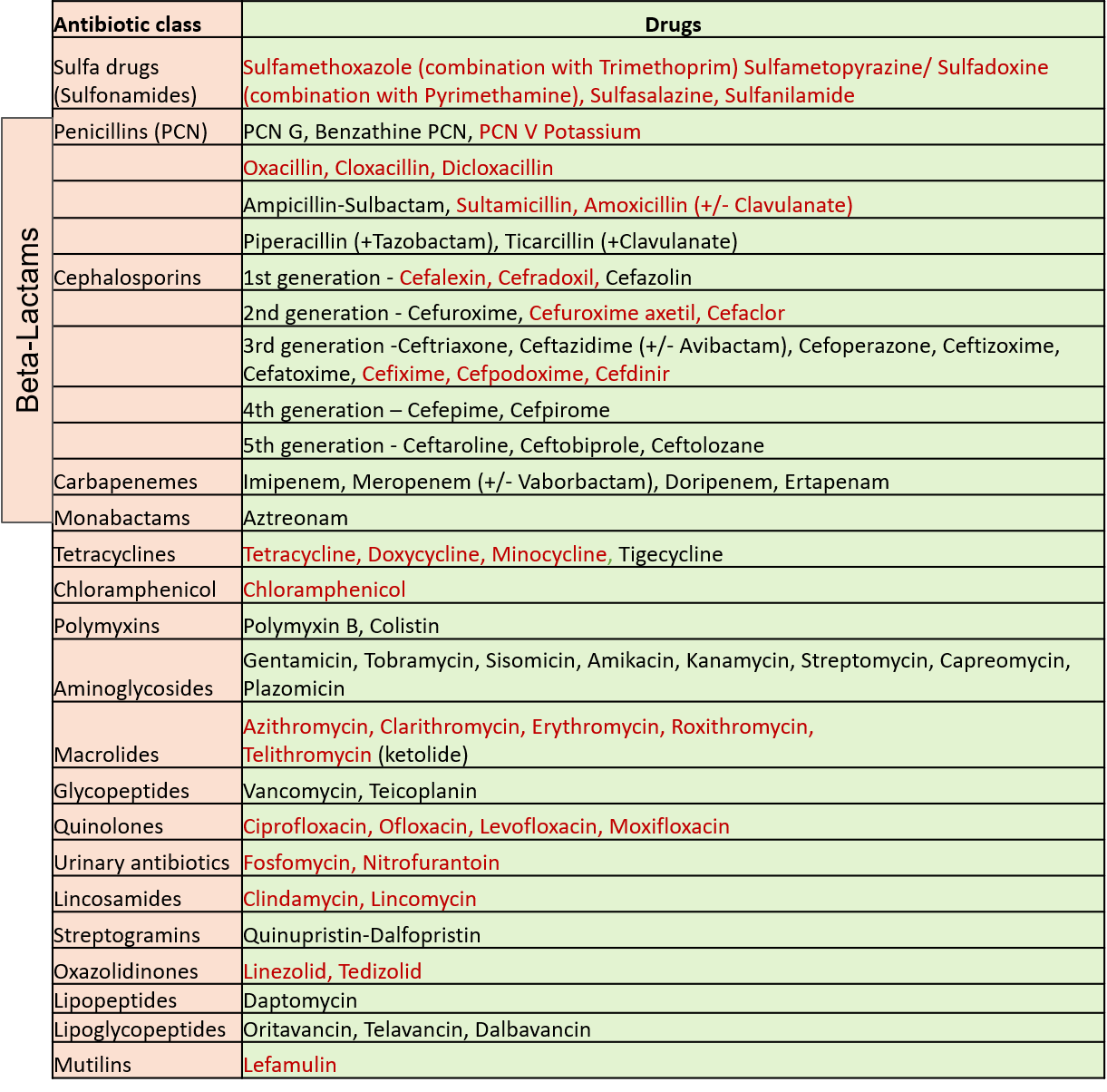
Antibiotics can be taken by the oral route (by mouth as tablets/capsules/syrups/granules), as injections, or by application on localized accessible sites (topical use- as eye/ear drops, ointments, creams, or powders). Those indicated in red in the table above can be taken orally. Some of these are available as both oral preparations and injections.
The first 3 members in the aminoglycoside and macrolide group, polymyxin B and the ones in the quinolones–lincosamide group are also available for topical use. Certain antibiotics can be given only by the local application route due to high toxicity if taken orally like nadifloxacin (quinolone group), neomycin, framycetin (aminoglycoside group), mupirocin, fusidic acid, bacitracin, and retapamulin (mutilin group).
Some antibiotics get concentrated in a particular body fluid (like urine – fosfomycin, nitrofurantoin) and therefore are mainly used for those infections (urinary tract infections).
WHAT IS RESISTANCE?
Resistance is when a micro-organism initially susceptible to an antibiotic is now not affected by it. This is a huge challenge that antibiotics today face due to large-scale usage. It’s a constant one-upmanship fight between humans and the bacteria and the bacteria seem to be way smarter! The bacteria develop different types of ‘skills’ to prevent the antibiotic from being effective on them- like pumps on their cell membrane to throw the antibiotic out or prevent entry, or slightly change/modify the enzymes, protein or target structure on which the antibiotic acts.
Bacteria also release enzymes that can inactivate or break the chemical structure of the antibiotic. Some of the beta-lactam group antibiotics can get inactivated by beta-lactamase or carbapenemase enzymes released by many bacteria. So now these groups of antibiotics are often combined with ‘beta-lactamase inhibitors’ (like amoxicillin plain versus the combination of amoxicillin with beta-lactamase inhibitor called clavulanate). Other beta-lactamase inhibitors sometimes combined with the beta-lactam antibiotics are sulbactam, tazobactam, avibactam and vaborbactam. Some of these like avibactam also inhibit carbapenemase. Extended-spectrum β-lactamases (ESBLs) are a group of diverse, complex and rapidly evolving enzymes that are posing a major therapeutic challenge today in the treatment of hospitalized and community-based infections caused by Klebsiella, E coli and Proteus. These enzymes share the ability to hydrolyze third-generation cephalosporins and aztreonam and exhibit co-resistance to many other classes of antibiotics, resulting in limitations in therapeutic options.
Some bacteria may be smarter than others and acquire one of the resistance mechanisms very fast. Many strains of bacteria like Staphylococcus, Streptococcus group, Klebsiella, Pseudomonas, and Acinetobacter along with TB-causing bacteria (Mycobacterium) and typhoid bacteria (Salmonella typhi) have shown a high degree of resistance to several antibiotics (MDR: Multidrug-resistant and XDR: Extensively drug-resistant) and are becoming tougher to treat with the need for newer antibiotics to be developed. Such resistant bacteria are often labeled by their resistance type like MRSA (methicillin-resistant Staphylococcus aureus), VRSA (vancomycin-resistant Staphylococcus aureus), ESBL Enterobacteriaceae, CRE (carbapenem-resistant Enterobacteriaceae) CRAB (Carbapenem-resistant Acinetobacter baumannii), etc.
The minimum concentration at which the antibiotic inhibits/kills the majority of a bacterial population is called the minimum inhibitory/lethal concentration (MIC or MLC) which is calculated for every antibiotic for each kind of bacteria. The dose and frequency of giving antibiotics are formulated on the basis of maintaining their concentration well above the MIC. Chances of resistance increase when bacteria are frequently exposed to antibiotics at a lower concentration, which is not enough to kill them but gives them an opportunity to start adapting to prevent the effect of that antibiotic on them. This makes each subsequent generation of the bacteria get more and more resistant to that antibiotic.
This is why more exposure of the community to an antibiotic, indiscriminate usage or overuse, extensive use in livestock, inappropriate duration and dose of use, and lack of hygienic practices and health regulations leads to the development of resistance of bacteria to many antibiotics. Most common infections like cold, cough and mild fever are caused by viruses and do not require antibiotics which are ineffective against viruses. However, antibiotics are often prescribed in practice for the same, contributing to the growing problem of resistance.
WAYS TO REDUCE ANTIMICROBIAL RESISTANCE (AMR)
Government and Health Authorities are striving across countries to incorporate plans, effective monitoring, and regulations to reduce the rising resistance menace. New antibiotics are always being researched and are in the pipeline, having novel mechanisms of action for development and use in resistant infections. However, reducing AMR is the responsibility not only of scientists and administrators but also of the medical practitioners and the common man. Some steps towards reducing AMR are as follows –
- Take antibiotics only with a doctor’s prescription after the clinical examination of your current condition. Do not use past prescriptions for similar symptoms to start antibiotics on your own.
- Most common colds, fevers and coughs are viral in nature and become okay in 3-5 days without any need or use of antibiotics. Rest, good nutrition and hydration along with simple lifestyle measures ensure recovery. Do not resort to or insist on using antibiotics expecting ‘fast’ results
- Always take the correct dosage strength as many times a day as mentioned on your prescription. Do not omit or modify dosage on your own.
- Complete the duration of the prescribed antibiotic course even if you feel completely recovered earlier.
- Do not share your antibiotics or give leftover antibiotics to family/community members.
- Do not medicate with more than one antibiotic at a time unless combinations are specifically prescribed by your doctor.
- Practice hygiene like proper waste disposal, hand washing, and maintaining clean surroundings to reduce infection rates. Make sure your vaccination schedule is up to date.
- Ensure a healthy nutritious diet at all times as this improves immunity for both preventing and fighting infections.
- Before starting antibiotics empirically, it is wise to have a sample taken from the infected site (cost and feasibility permitting in developing countries), to test for the causative bacteria and its sensitivity to various antibiotics.
- Regular notification of resistance patterns to health authorities by doctors helps to regulate usage and select appropriate antibiotics for that region.
SIDE EFFECTS OF ANTIBIOTICS
Antibiotics given orally can also destroy the ‘good friendly bacteria’ in our gut which help in digestion and also produce some vitamins. Therefore, indigestion, nausea, cramps and diarrhea are known side effects of many antibiotics. So taking plenty of curd, or supplements called ‘probiotics’ (deliver friendly bacteria back to the gut) along with the antibiotics is often recommended. Adding a multivitamin supplement is also beneficial.
Allergic reactions may be seen to some antibiotics usually presenting after the first or second dose. Local application of antibiotics can show occasional skin irritation, burning or redness. Some can have different side effects like rashes, lethargy, photosensitivity, skin/tooth discolorations, drug fevers, changes in laboratory parameters and blood counts. Most of these are reversible on discontinuation. However, it’s best to inform the doctor if facing any disturbing side effects.
One has to be very careful before giving antibiotics to children, as some antibiotics can cause growth stunting (quinolones) and are not indicated in children. Dosages may need to be adjusted especially in elderly who have compromised kidney or liver function. Some antibiotics may interact with other medicines and supplements being taken so such information needs to be shared with the doctor.
NEW ANTIBIOTICS IN THE PIPELINE
These are antibiotics in the research phase showing promise, but still awaiting human trials. Clovibactin, isolated from a bacterium found in sandy soil., acts by targeting three peptidoglycan precursors essential for bacterial cell wall production, while Teixobactin interferes with two steps in the cell-wall assembly of the targeted bacteria. Both these antibiotics target Gram-positive bacteria, like MRSA. Zosurabalpin can kill the drug-resistant bacterium Acinetobacter baumannii (CRAB) by inhibiting the transport of lipopolysaccharide (LPS), which is essential for the cell wall structure of Gram-negative bacteria.
Also read:
Skin Infections – Types, Predisposing Factors and Health Measures
For any query, additional information or to discuss any case, write to info@drvarsha.com, and be assured of a response soon.
References
Antimicrobial resistance in the environment: The Indian scenario – Indian Journal of Medical Research 2019, 145(2):119
National Action plan for antibiotic resistance: India. J Family Med Prim Care. 2019 Jun; 8(6): 1828–1834