AIDS (Acquired Immunodeficiency Syndrome) is caused by infection with HIV (Human Immunodeficiency Virus).
HIV is of two types 1 and 2. Type 2 is less infective and virulent, shows less vertical (mother to child) and heterosexual spread, has lower genetic diversity and prevalence (mainly in West Africa), and a much longer time to development of AIDS (>20 years vs <10 years) as compared to HIV 1 that is globally more common.
World AIDS Day was first observed on 1st December 1988, to bring greater awareness to HIV. It is also regarded by some as the longest-running disease awareness initiative of its kind in the history of public health. Shared here are some facts that would drive the global mission of ending AIDS by 2030.
MYTHS AND FACTS
- Life expectancy of HIV-infected people is poor
FACT: Life expectancy for people living with HIV (PLHIV) receiving proper care increased significantly from 1996 on. In 1996, the total life expectancy for a 20-year-old person with HIV was 39 years. In 2011, the total life expectancy increased to about 70 years. With the right treatment and care, people who have a good response to HIV treatment have excellent long-term prospects with similar life expectancy to HIV-negative peers.
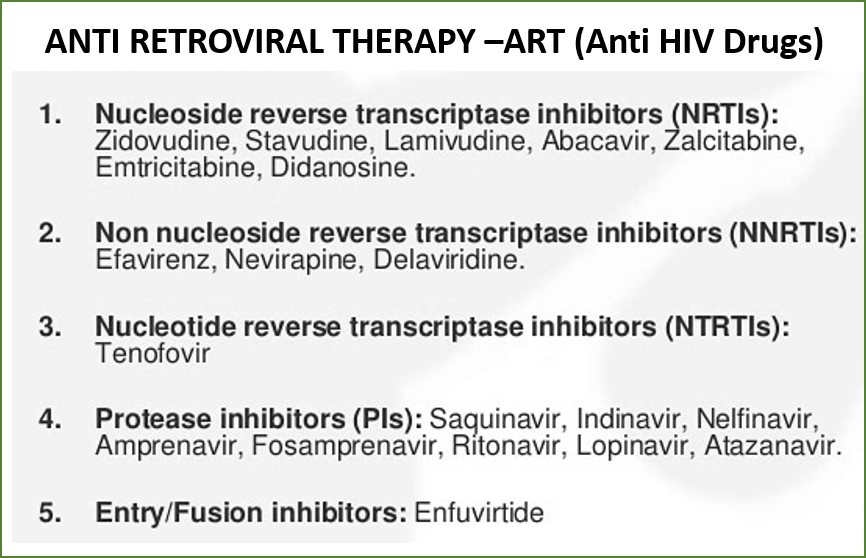


- ART initiation is indicated if CD4 is ≤500 cells/mm3.
FACT: Criteria for ART (antiretroviral therapy or anti-HIV drugs) initiation has evolved significantly over the years. As per previous guidelines, ART was initiated when CD4 (T helper white blood cells of our immune system) declined to certain levels e.g., <500 cells/mm3, or with a certain clinical stage of the disease. Based on current WHO recommendations, India has adopted the test-and-treat policy in 2017 regardless of clinical stage or CD4 count as evidence suggests that early ART initiation versus waiting for the CD4 count to decline to certain levels was associated with reduced morbidity, mortality, and HIV transmission.
- ART initiation is preferred and deferred as per age and population
FACT: All persons diagnosed with HIV infection are eligible for ART initiation regardless of CD4 count or WHO Clinical Staging, any age (Adults, Adolescents, Children), or any population (Pregnant/Migrant/others).
- In India currently, the HIV burden is negligible
FACT: India still has the world’s second-largest number 2.47 million people living with HIV (PLHIV). Yes, Overall, India’s HIV epidemic is slowing down. Between 2010 and 2019 new infections came down by 37% and AIDS-related mortality more than 66%. But still, there is a lot to catch up on, as due to a number of issues including HIV-related discrimination, relatively low levels of status awareness among PLHIV, and poor links between diagnosis and treatment, the mean progress is not as rapid as expected. One of the major reasons why we are unable to meet the needs of PLHIVs is the difficulty faced in designing the tailor-made program due to the lack of data on key populations and on certain key indicators such as viral suppression rates.
- Drug therapy for HIV is very complicated
FACT: As per WHO guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, tenofovir disoproxil fumarate (TDF) 300mg+ lamivudine (3TC) 300mg + dolutegravir (DTG) 50mg, known as the TLD regimen, is the preferred first-line antiretroviral therapy for adults and adolescents. Scientific evidence and programmatic experience have accumulated on the use of dolutegravir (DTG) in both first- and second-line ART (TLD regimen), including during pregnancy and tuberculosis co-treatment, and for children.
The TLD regimen tenofovir disoproxil fumarate (TDF) 300mg + lamivudine (3TC) 300mg + dolutegravir (DTG) 50mg as a fixed-dose combination offers harmonization of treatment for all adults, adolescents (age >10 years and weight >30kg), pregnant women and those with HIV-TB and HIV-Hepatitis B co-infections. It also offers the advantage of decentralized service delivery and monitoring. It is a simple, potent, and well-tolerated regimen.
The World Health Organization’s (WHO) latest report highlights high levels of HIV viral load suppression (>90%) in populations receiving dolutegravir (DTG)-containing antiretroviral therapy (ART). However, among the surveys reported, levels of resistance to dolutegravir ranged from 3.9% to 8.6%, and reached 19.6% among people experienced with treatment and who transitioned to a DTG-containing ART while having high HIV viral loads. So, there is an urgent need for increased vigilance and surveillance of HIV drug resistance.
Alternate regimens are:
Abacavir (ABC) 600mg + Lamivudine (3TC) 300mg one tablet, + DTG (50 mg) once daily: For PLHIV with body weight <30 kg.
ABC 600 mg once daily + Lamivudine (as per creatinine clearance) and DTG 50 mg once daily: for all patients with high (above the upper limit of normal-ULN) for lab serum creatinine values (creatinine clearance to be calculated).
TDF (300 mg) + Lamivudine (300 mg) + DTG (50 mg) once daily (that is TLD regimen in the evening) with the addition of DTG 50 mg (morning): for PLHIV on Rifampicin containing antitubercular therapy (ATT) regimen (this additional DTG dose to be stopped after completion of Rifampicin based ATT)
TLE regimen [If efavirenz is contraindicated (HIV2 /HIV 1 & 2 /prior NNRTI drugs exposure) then TL (TDF-lamivudine) +LPV/r (lopinavir–ritonavir) (TL- once daily, LPV/r -2 twice daily)]: Females in the reproductive age group who opt out of TLD.
- DTG has a higher genetic resistance barrier.
FACT: This is true. DTG is linked to a more rapid viral suppression and higher genetic resistance barrier when compared with NNRTIs. Systematic reviews and metanalysis conducted by WHO have shown that when compared with efavirenz, DTG-based regimens are better tolerated and tend to be protective against treatment discontinuation due to adverse events. Among stable, virologically suppressed patients on NNRTI or PI group of drugs, substitution with a DTG-containing regimen was also well-tolerated and non-inferior in maintaining viral suppression, with high rates of satisfaction compared to those remaining on their existing regimen.
- DTG is not recommended for women of child-bearing age group
FACT: The risk-benefit models suggest that the benefits of DTG for this group newly initiating ART, are likely to outweigh the risks associated with it. Benefits include greater maternal viral suppression, fewer maternal deaths, fewer sexual transmissions, and fewer mother-to-child transmissions of HIV. Risks include adult morbidity resulting from DTG-associated weight gain and neonatal deaths among the infants of pregnant women with DTG-associated weight gain. Blanket exclusions that deny women equitable access to this optimal HIV treatment (with DTG) are not warranted or justified.
Effective contraception should be offered to adult women and adolescent girls of childbearing age or potential. DTG can be prescribed for adult women and adolescent girls of childbearing age or potential who wish to become pregnant or who are not otherwise using or accessing consistent and effective contraception if they have been fully informed of the potential increase in the risk of neural tube defects. In case a woman does not wish to initiate TLD after adequate counseling, she should be initiated on a TLE regimen. If the woman is identified to be pregnant after the first trimester, DTG should be initiated or continued for the duration of the pregnancy.
- CD4 is the preferred approach to monitor PLHIV on ART
FACT: In 2013, WHO recommended viral load as the preferred approach for monitoring HIV1-infected individuals on ART over immunological (CD4) and clinical monitoring. This is because viral load provides an early and more accurate indication of treatment failure and the need to switch to second-line therapy. This helps reduce the accumulation of drug-resistance mutations and improves clinical outcomes for PHLIV on ART. Measuring viral load can also help distinguish between treatment failure and non-adherence.
HIV-2 infected individuals should not be monitored by viral load but by assessing CD4 count.
- COVID vaccination is not advisable for PLHIV as they are already immune-compromised.
FACT: For PLHIV, COVID-19 vaccines carry the same benefits as they carry to all individuals and communities that is the prevention of severe disease due to SARS-CoV-2 and potentially reduced transmission of the SARS-CoV-2 virus. The COVID-19 vaccines authorized by regulators significantly reduce the risk of severe disease and death from COVID in PLHIV.
- In India, health insurance coverage for PLHIV is very limited.
FACT: Unfortunately, this is true. There are limited health insurance plans that cover HIV or AIDS treatment in India. Unlike other conditions, HIV/ AIDS as a pre-existing condition is not covered even after enduring the waiting period. It remains an exclusion, now and always.
The author Dr. Bhawana Shinde (MBBS, MD-pharma) is an HIV Expert and Consultant (HIV), at the University of Washington’s I-TECH, India. She has been working in the field of HIV-AIDS for several years including handling medical affairs and research with Pharmaceuticals. Apart from HIV, Dr. Bhawana has also done notable work in the field of autism and parenting. Contact: bhawana83shinde@gmail.com
REFERENCES:
Also read:
Tuberculosis (TB): Awareness, Diagnosis, Treatment, and Prevention


